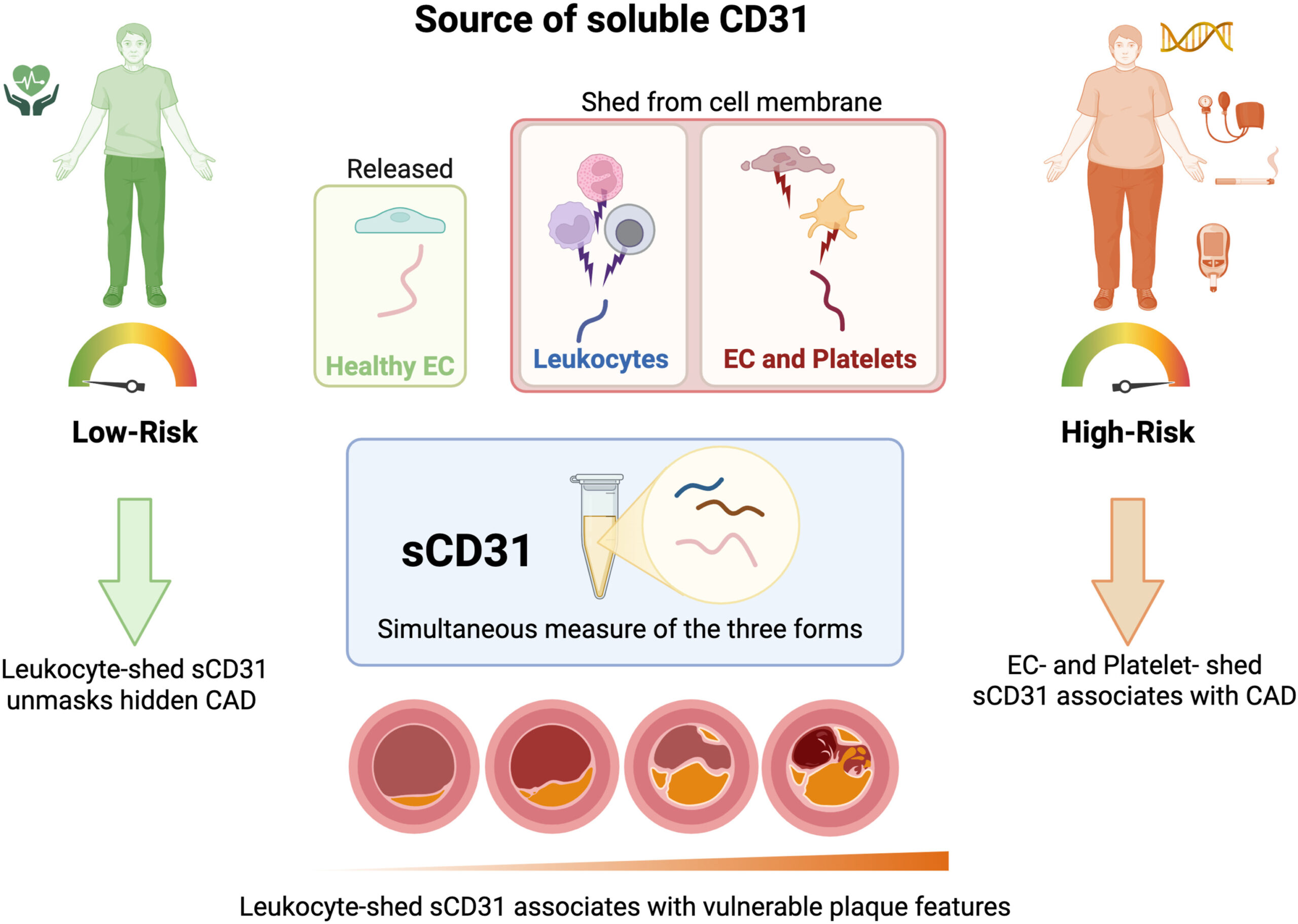
Leukocyte-shed soluble CD31 unmasks coronary disease in low-risk outliers and provides source-specific inflammatory signatures of vulnerable plaques
Marco Magnonia,1, Daniele Andreini b,c,1, Felicita Andreotti d,e, Roberto Latini f, Attilio Maseri g,2, Antonino Nicoletti h, Aldo Pietro Maggioni g,1, Giuseppina Caligiuri h,i,
Background and aim: Leukocyte activation has been linked to coronary atherosclerotic disease and may contribute to inflammatory processes associated with vulnerable plaques. We investigated different forms of soluble CD31 (sCD31) and their relationship with coronary artery disease (CAD) and plaque characteristics in patients from the “Coronary Atherosclerosis in outlier subjects: Protective and novel Individual Risk factor Evaluation” (CAPIRE) study, with specific focus on risk-dependent patterns.
Methods: We measured different sCD31 forms in plasma from 544 individuals undergoing coronary computed tomography angiography (CCTA). We distinguished between transmembraneless full-length sCD31 (endothelial cells), short sCD31 (leukocyte-shed), and long sCD31 (platelet-derived) using a custom cytometric bead assay with epitope-mapped monoclonal antibodies We analyzed these markers’ associations with CAD presence, plaque characteristics, and traditional risk factors, adjusting leukocyte-shed CD31 for IL-6 levels to control for systemic inflammation.
Results: The different forms of sCD31 demonstrated distinct risk-associated patterns. In low-risk individuals, total sCD31 showed a significant negative association with CAD, while leukocyte-shed sCD31 demonstrated a strong positive association. Conversely, in high-risk patients, this pattern reversed: total sCD31 exhibited a positive association with CAD, while the non-leukocyte component showed a negative association. Leukocyte-shed sCD31 addition to prediction models improved discriminatory power, especially in low-risk populations (AUC: 0.79 → 0.95).
Conclusions: Leukocyte-shed sCD31 unmasks coronary disease in low-risk outliers and provides source-specific inflammatory signatures of vulnerable plaques. The differential pattern of sCD31 forms based on risk burden suggests distinct pathophysiological mechanisms driving atherosclerosis in different patient populations, addressing a critical gap in current risk assessment.