Thrombopoietin-Dependent Myelo-Megakaryopoiesis Fuels Thromboinflammation and Worsens Antibody-Mediated Chronic Renal Microvascular Injury
Douté M, Sannier A, Even G, Tran T, Gaston AT, Delbosc S, Loyau S; Bruneval P, Witko-Sarsat V, Mouthon L, Nicoletti A, Caligiuri G, Clement M
Background
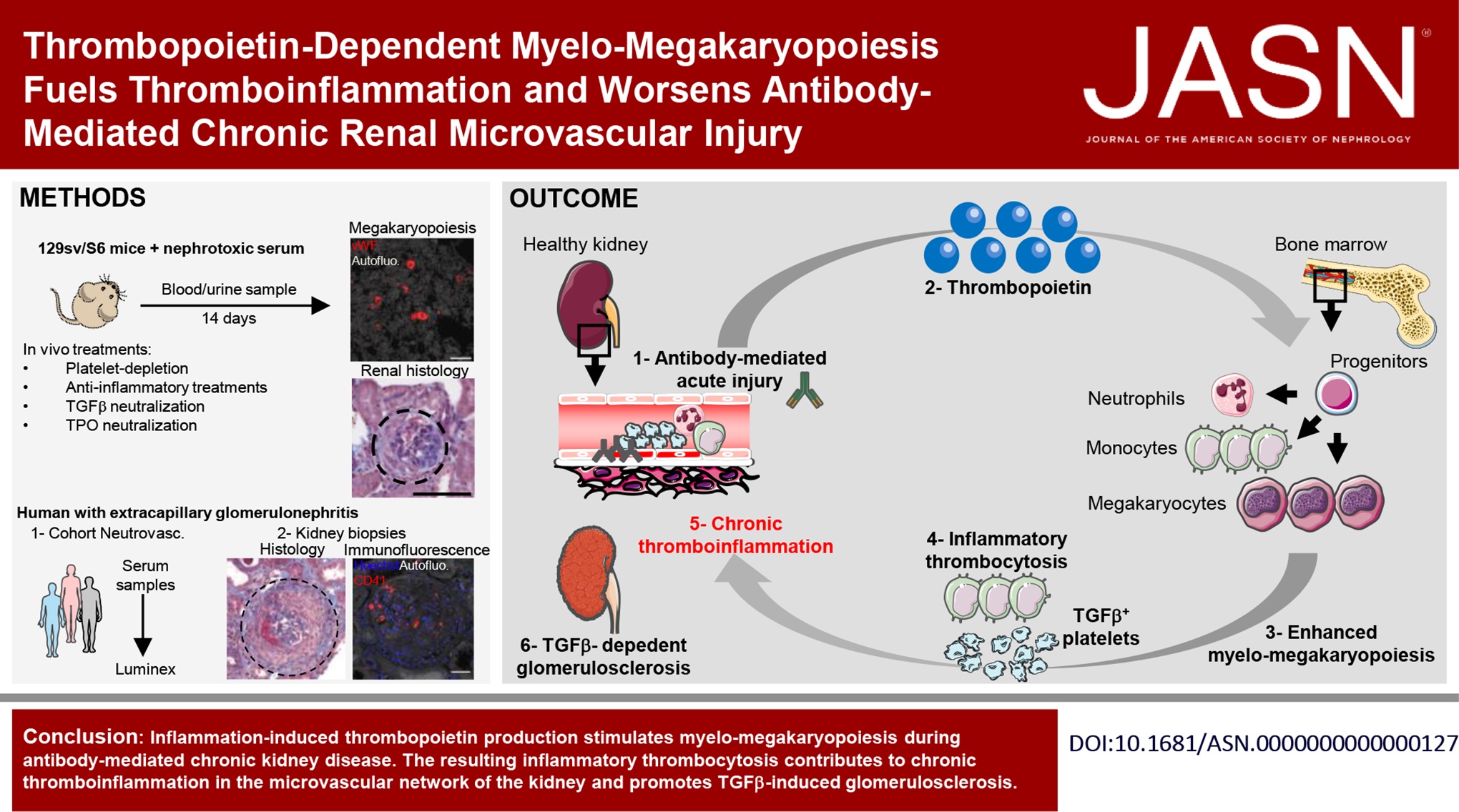
Chronic thromboinflammation provokes microvascular alterations and rarefaction, promoting organ dysfunction in individuals with various life-threatening diseases. Hematopoietic growth factors (HGFs) released by the affected organ may sustain emergency hematopoiesis and fuel the thromboinflammatory process.
Methods
Using a murine model of antibody-mediated chronic kidney disease (AMCKD) and pharmacological interventions, we comprehensively monitored the response to injury in the circulating blood, urine, bone marrow, and kidney.
Results
Experimental AMCKD was associated with chronic thromboinflammation and the production of HGFs, especially thrombopoietin (TPO), by the injured kidney, which stimulated and skewed hematopoiesis toward myelo-megakaryopoiesis. AMCKD was characterized by vascular and kidney dysfunction, TGFβ-dependent glomerulosclerosis, and microvascular rarefaction. In humans, extracapillary glomerulonephritis is associated with thromboinflammation, TGFβ-dependent glomerulosclerosis, and increased bioavailability of TPO. Analysis of albumin, HGF, and inflammatory cytokine levels in sera from patients with extracapillary glomerulonephritis allowed us to identify treatment responders. Strikingly, TPO neutralization in the experimental AMCKD model normalized hematopoiesis, reduced chronic thromboinflammation, and ameliorated renal disease.
Conclusion
TPO-skewed hematopoiesis exacerbates chronic thromboinflammation in microvessels and worsens AMCKD. TPO is both a relevant biomarker and a promising therapeutic target in humans with CKD and other chronic thromboinflammatory diseases.