Cleaved CD31 as a target for in vivo molecular imaging of inflammation
Vigne J, Bay S, Aid-Launais R, Pariscoat G, Rucher G, Sénémaud J, Truffier A, Anizan N, Even G, Ganneau C, Andreata F, Le Borgne M, Nicoletti A, Le Guludec D, Caligiuri G & Rouzet F
2019 • Scientific Reports • [pdf]
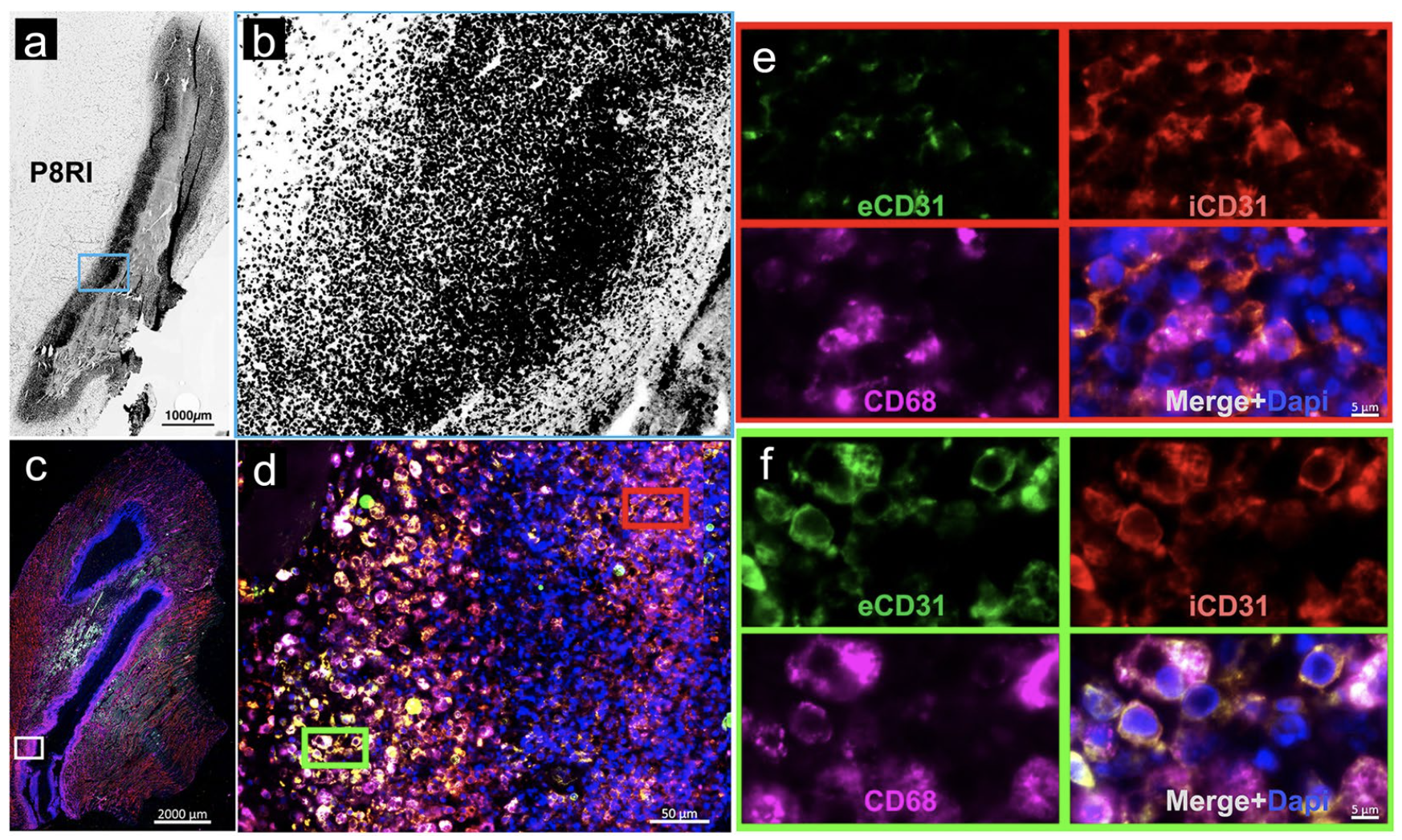 There is a need for new targets to specifically localize inflammatory foci, usable in a wide range of organs. Here, we hypothesized that the cleaved molecular form of CD31 is a suitable target for molecular imaging of inflammation. We evaluated a bioconjugate of D-P8RI, a synthetic peptide that binds all cells with cleaved CD31, in an experimental rat model of sterile acute inflammation. Male Wistar rats were injected with turpentine oil into the gastrocnemius muscle two days before 99m Tc- HYNIC-D-P8RI (or its analogue with L-Proline) SPECT/CT or [ 18 F]FDG PET/MRI. Biodistribution, stability study, histology, imaging and autoradiography of 99m Tc-HYNIC-D-P8RI were further performed.
There is a need for new targets to specifically localize inflammatory foci, usable in a wide range of organs. Here, we hypothesized that the cleaved molecular form of CD31 is a suitable target for molecular imaging of inflammation. We evaluated a bioconjugate of D-P8RI, a synthetic peptide that binds all cells with cleaved CD31, in an experimental rat model of sterile acute inflammation. Male Wistar rats were injected with turpentine oil into the gastrocnemius muscle two days before 99m Tc- HYNIC-D-P8RI (or its analogue with L-Proline) SPECT/CT or [ 18 F]FDG PET/MRI. Biodistribution, stability study, histology, imaging and autoradiography of 99m Tc-HYNIC-D-P8RI were further performed.
Biodistribution studies revealed rapid elimination of 99m Tc-HYNIC-D-P8RI through renal excretion with almost no uptake from most organs and excellent in vitro and in vivo stability were observed. SPECT/ CT imaging showed a significant higher 99m Tc-HYNIC-D-P8RI uptake compared with its analogue with L-Proline (negative control) and no significant difference compared with [ 18 F]FDG (positive control).
Moreover, autoradiography and histology revealed a co-localization between 99m Tc-HYNIC-D-P8RI uptake and inflammatory cell infiltration. 99m Tc-HYNIC-D-P8RI constitutes a new tool for the detection and localization of inflammatory sites. Our work suggests that targeting cleaved CD31 is an attractive strategy for the specific in vivo imaging of inflammatory processes.

