Enabling MedTech Translation in Academia: Redefining Value Proposition with Updated Regulations
Letourneur D, Joyce K, Chauvierre C, Bayon Y, Pandit A
2020 • Adv Healthc Mater• [pdf]
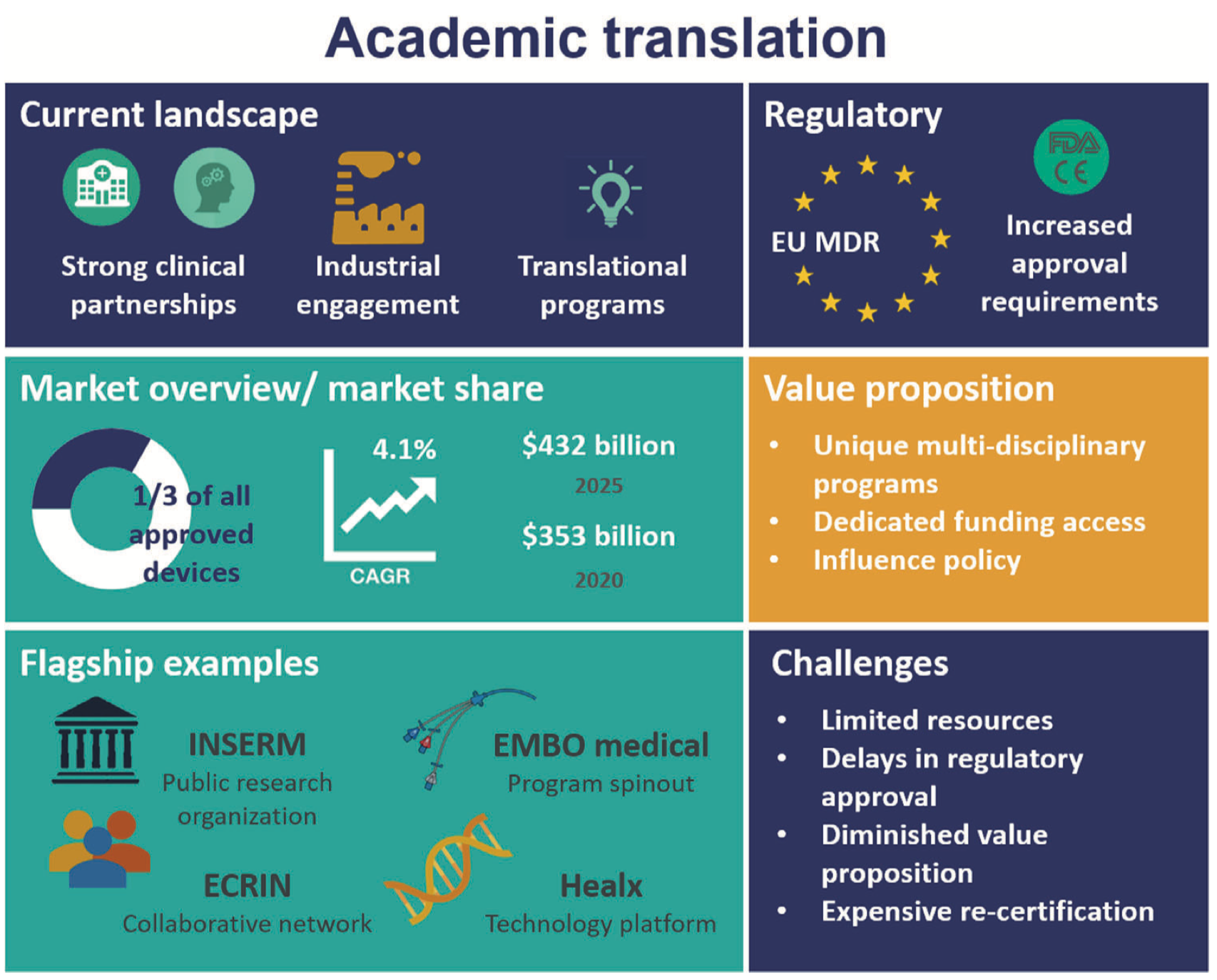
Academic institutions are becoming more focused on translating new technologies for clinical applications. A transition from “bench to bedside” is often described to take basic research concepts and methods to develop a therapeutic or diagnostic solution with proven evidence of efficacy at the clinical level while also fulfilling regulatory requirements. The regulatory environment is evolving in Europe with transition and grace periods for the full enforcement of the Medical Device Regulation 2017/745 (MDR), replacing the Medical Device Directive 93/42/EEC (MDD). These new guidelines increase demands for scientific, technical, and clinical data with reduced capacity in regulatory bodies creating uncertainty in future product certification. Academic translational activities will be uniquely affected by this new legislation. The barriers and threats to successful translation in academia can be overcome by strong clinical partnerships, close-industrial collaborations, and entrepreneurial programs, enabling continued product development to overcome regulatory hurdles, reassuring their foothold of medical device development.

