Downstream Microvascular Thrombosis in Cortical Venules Is an Early Response to Proximal Cerebral Arterial Occlusion
Desilles JP, Syvannarath V, Di Meglio L, Ducroux C, Boisseau W, Louedec L, Jandrot-Perrus M, Michel JB, Mazighi M, Ho-Tin-Noe B
2018 • J Am Heart Assoc • [pdf]
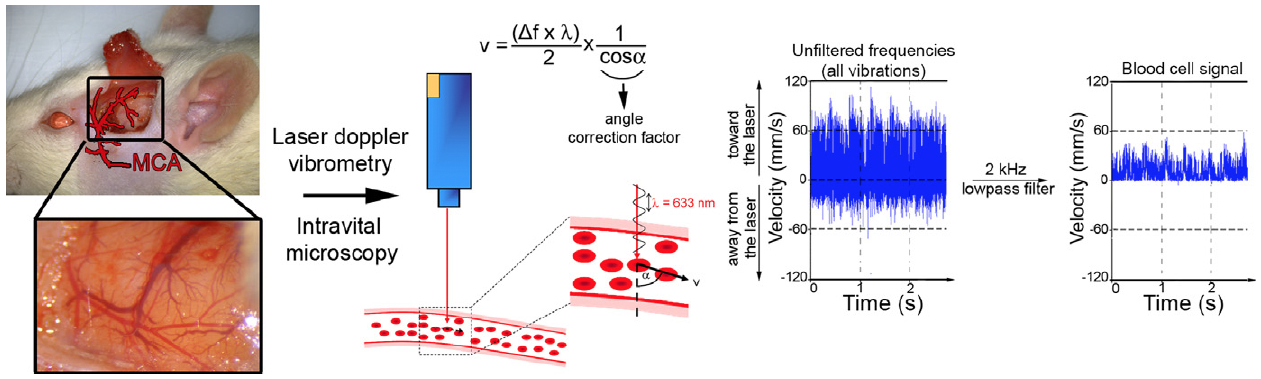
BACKGROUND: Previous experimental studies have shown that downstream microvascular thromboinflammation is involved in brain damage from acute ischemic stroke. Using intravital microscopy, we investigated and characterized the sequence of downstream microvascular thromboinflammation in an ischemia/reperfusion acute ischemic stroke model.
METHODS AND RESULTS: Rats underwent transient monofilament middle cerebral artery (MCA) occlusion. Cerebral microcirculation in the MCA territory was exposed through a craniotomy and analyzed using real-time intravital imaging coupled with laser Doppler interferometry. Leukocytes, platelets, fibrinogen, and blood-brain barrier permeability were analyzed by intravenous injection of fluorescent antibodies and bovine serum albumin. MCA occlusion induced a sudden and profound drop in downstream microvascular blood flow associated with leukocyte margination in the venous compartment. Leukocyte margination fostered fibrinogen deposition and thrombosis in postcapillary venules. Either in venules or arterioles, blood flow was not fully restored after MCA recanalization. Furthermore, venular thrombi persisted despite MCA recanalization, and leukocyte extravasation continued to develop in venules in association with blood-brain barrier disruption. Finally, microhemorrhages were occasionally observed, colocalizing with thrombosed venules characterized by marked leukocyte margination.
CONCLUSIONS: We showed that microvascular thrombosis in transient monofilament MCA occlusion and blood-brain barrier disruption are initiated immediately after occlusion and are propagated through the venous compartment in close association with marginating leukocytes. MCA occlusion-induced downstream microvascular thromboinflammation response was responsible for incomplete reperfusion after MCA recanalization and delayed microhemorrhages.

