Radical Copolymerization of Vinyl Ethers and Cyclic Ketene Acetals as a Versatile Platform to Design Functional Polyesters
Tardy A, Honore JC, Tran J, Siri D, Delplace V, Bataille I, Letourneur D, Perrier J, Nicoletti C, Maresca M, Lefay C, Gigmes D, Nicolas J, Guillaneuf Y
2017 • Angew Chem Int Ed Engl • [pdf]
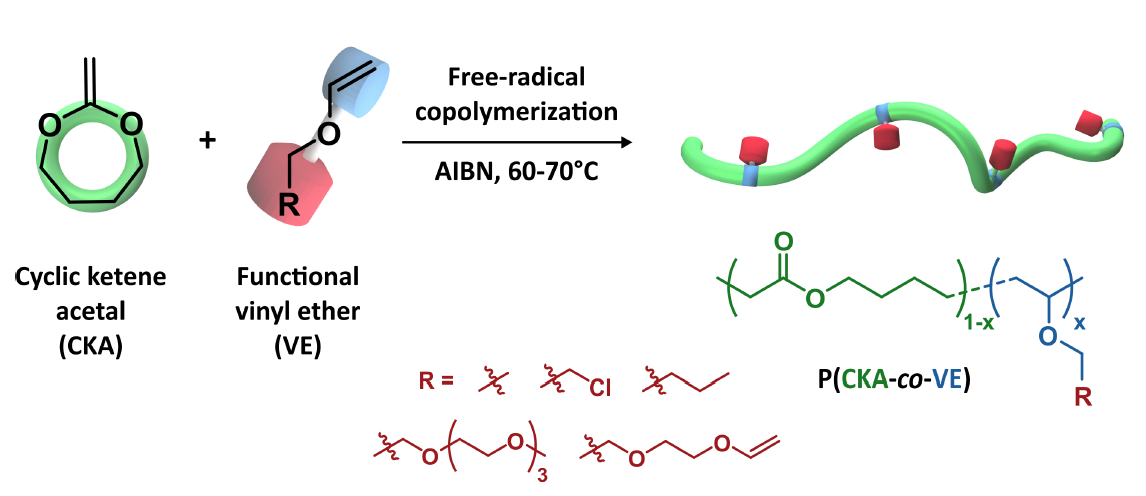
For the first time, the free-radical copolymerization of cyclic ketene acetals (CKAs) and vinyl ethers (VEs) was investigated as an efficient, yet simple approach for the preparation of functional aliphatic polyesters. The copolymerization of CKA and VE was first predicted to be quasi-ideal by DFT calculations. The theoretical prediction was experimentally confirmed by the copolymerization of 2-methylene-1,3-dioxepane (MDO) and butyl vinyl ether (BVE), leading to rMDO = 0.73 and rBVE = 1.61. We then illustrated the versatility of this approach by preparing different functional polyesters: (i) copolymers functionalized by fluorescent probes; (ii) amphiphilic copolymers grafted with poly(ethylene glycol) (PEG) side chains able to self-assemble into PEGylated nanoparticles; (iii) antibacterial films active against Gram+ and Gram- bacteria (including a multiresistant strain) and (iv) cross-linked bioelastomers with suitable properties for tissue engineering applications.